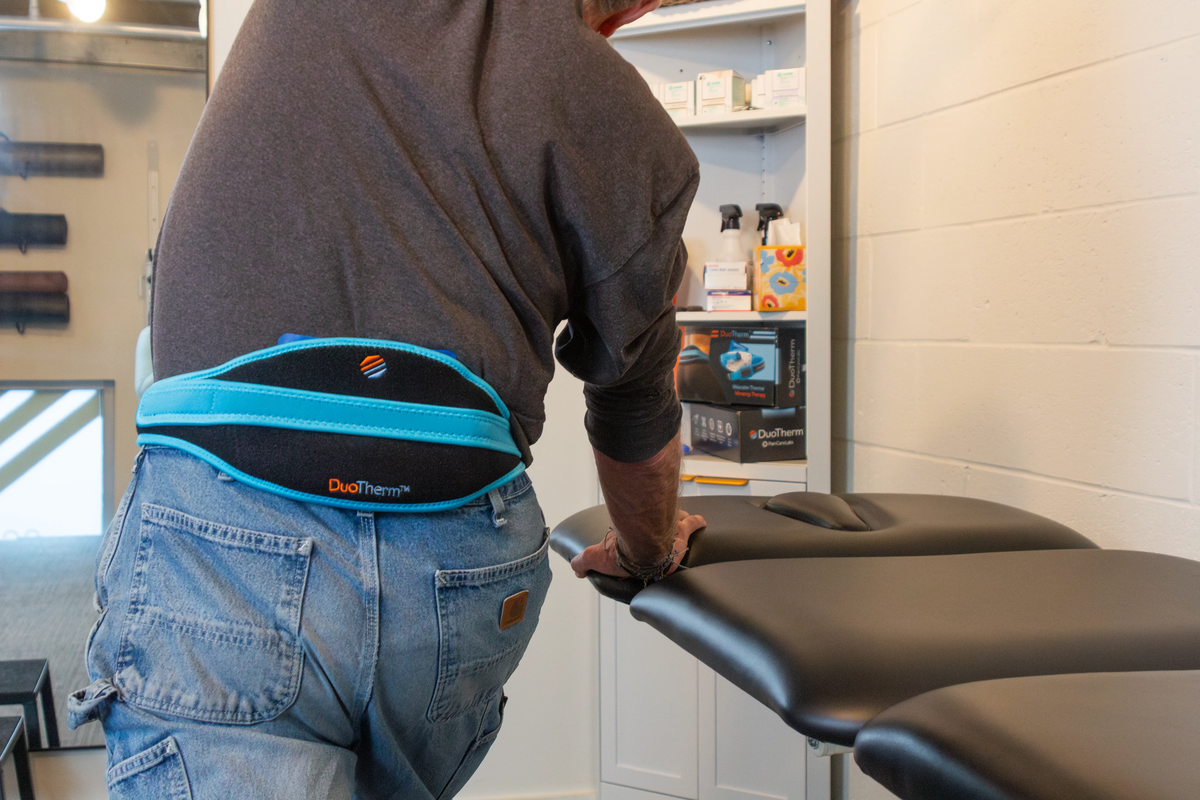
DuoTherm
Noninvasive thermomechanical medical device
Multimodal compression pain relief with eight noninvasive neuromodulation therapy cycles, acupressure, and soothing heat or anti-inflammatory cold.
“This device was the only thing that knocked my pain all the way down to zero.”
“Prior to using DuoTherm I was having trouble running, sitting at work, and participating in activities of daily living. Now after consistent use, I am back to training for a 1/2 marathon, and most of the time pain free. ”
“Great product! Finally something that works without pain killers!”
Regulatory Path to Market
Clinical Trial History
NCT04494841: FDA NIH Pilot N=20 Milestone >50% reduction in pain intensity
FDA NIH Pivotal Trial with Active Control LG 8-Channel TENS in moderate-to-severe LBP
NCT04491175: Reduce Opioid prescribing 30% and Use past 7 days in the opioid naive.
NCT04494698: Significantly reduce acute pain intensity and restore function for chronic pain using PROMIS Pain Interference.

Testimonials
“It has been the capstone to my 30 year healing journey while struggling with chronic back pain. Once I started using my DuoTherm, I quickly noticed my morning pain and stiffness reduced. After any hard physical exercise, I am able to snap myself out of the “pain cycle” that I would typically fall into once I stopped moving.”
“I used Duotherm for pain management after my abdominal surgery. It was exactly what I needed to get me through the time in between ibuprofen doses and helped me fall asleep on rougher days. I was able to limit my at-home opioid usage to only three doses and relied on over-the-counter options for the rest of my multi-month recovery.”
“My chronic low back pain and associated sciatica was debilitating before using Duotherm. I tried everything from PT to injections but could not control the pain without daily use of pain meds. I was up to 900 milligrams of Gabepentin as well as taking NSAIds, but I still could not sit for more than 10 minutes at a time or sleep at night. This was not sustainable. I started using Duotherm 3-4 times a day and after a few weeks was able to get a full nights rest and am now able to sit in a car or plane for hours without pain and without the meds. I was recently able to travel to Patagonia for an active vacation with my husband for our 40th wedding anniversary without worrying about the pain I used to get from travel. I am now able able to hike, ride horses, enjoy gardening and get a good nights sleep without the risk of taking daily pain meds! I credit DuoTherm for my new lease on life!”
Initial R&D conducted as part of an Indiegogo campaign. Reviews are from original investors who received prototype devices, and compassionate use recipients prior to FDA indications and review.